|
|
![]()
What is Electroless Gold Plating ?
Electroless means that the process used to plate the gold onto a surface is not the traditional electroplating cell having electrodes and an external power supply. Electroless gold is deposited from a solution purely as the result of the reaction of chemicals in the bath. The electroless plating bath is comprised of several of the same items found in an electrolytic gold plating bath, but it also includes chemicals (reducing agents) to provide electrons for the reaction and other chemicals (chelating agents and stabilizers) to control the reaction so that it can be predictable and controllable as a manufacturing process.
Why Does It Matter Which Process is Used to Plate ?
Is the Deposit the Same ?
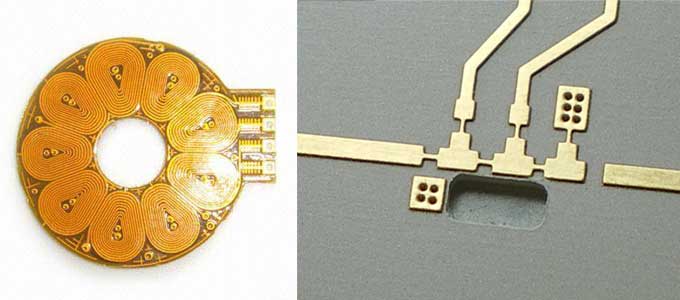
Using electroless gold plating, circuits can be designed and fabricated in their final configuration. This saves the manufacturer several steps but more importantly it allows the circuit design to place primary consideration on optimum circuit performance and minimum circuit size.
The electroless gold deposit is identical to an electrolytic pure gold deposit (i.e., Mil-G-45204 C Type III or ASTM B488 Type 1 hardness code A) in essentially every way; thickness, purity, hardness, grain size, density and color. The primary difference from an electrolytic gold deposit is it's second distinct advantage... improved thickness distribution.
Electrolytic plating creates deposits that have thickness variations resulting primarily from the shape of the electrical field between the cathode surfaces and the anode. These deposits are thicker on corners and edges and surfaces where the distance to the anode is shortest. They are also thinner in the middle of surfaces, inside of holes and on surfaces facing away from the anode.
Electroless plating completely eliminates this source of variation. Electroless gold plated deposits are uniform in thickness around corners, on both sides of a part, even on the inside walls of small holes that may be very deep.